Assessment of Haematological Parameters in Drug-Resistant TB
DOI:
https://doi.org/10.51173/jt.v5i1.1082Keywords:
Mutation, Rpob Gene, Drug Resistance, Mycobacterium TuberculosisAbstract
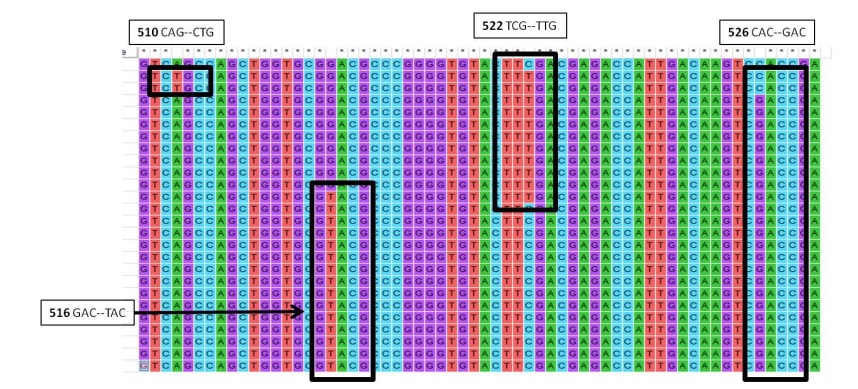
Mycobacterium tuberculosis causes tuberculosis (TB), the world's deadliest infectious illness. In addition to the lungs, bone marrow is also impacted by tuberculosis. Significant haematological abnormalities can be found in TB patients. This means that these haematological markers can be used to assess a patient's diagnosis, prognosis, and treatment outcome. The goals of this study are (a) to assess the hematological characteristics of TB patients, and (b) to examine the impact of rifampin (RIF) resistance on the prevalence of mutations in the whole rpoB gene of Mycobacterium tuberculosis. Fifty people participated in the study after being chosen through a systematic random sample process. From each participant, about 4 milliliters of blood were taken from a vein and estimated for the haematological parameters like Hb, WBC, Lymphocyes, neutrophils, Erythrocyte sedimentation rate and platelet count. Fifty tuberculosis isolates had their whole rpoB genes sequenced so that we could analyze the positions of the codons and the frequency with which they occurred. When compared to healthy controls, the values of hemoglobin and other blood indices were significantly lower or abnormal (p<0.05). ESR values were alarmingly increased in the subjects along with platelets (P value < 0.05). All of our 25 isolates exhibited four types of mutations at four RRDR positions (codons 510, 516, 522 and 526). We found codons 526 showed a high level (92%) of RIF resistance or mutations when compared to other positions. An easy and affordable way to forecast the progression of the illness and keep track of complications in underdeveloped nations is to test the haematological parameters of tuberculosis patients. RIF resistance is linked to certain mutations in the rpoB gene that may impact how RpoB and RIF interact. These results may be used to create new antibiotics and create cutting-edge diagnostic tools for TB medication resistance.
Downloads
References
Shafee M, Abbas F, Ashraf M, Alammengal M, Kakar N, Ahmad Z, et al. Hematological profile and risk factors associated with pulmonary tuberculosis patients in Quetta, Pakistan. Pak J Med Sci 2014;30:36 40. 2.
Kahase D, Solomon A, Alemayehu M. Evaluation of peripheral blood parameters of pulmonary tuberculosis patients at St. Paul’s hospital millennium medical college, Addis Ababa, Ethiopia: Comparative study. J Blood Med 2020;11:115 121.
Peresi E, Silva SM, Calvi SA, Marcondes Machado J. Cytokines and acute phase serum proteins as markers of inflammatory regression during the treatment of pulmonary tuberculosis. J Bras Pneumol2008;34:942 9.
Shaikh MK, Samo JA, Devrajani BR, Shah SZA, Shaikh S, Shaikh I. C reactive protein in patients with pulmonary tuberculosis. World Appl Sci J 2012;17:140 4.
Banerjee M, Chaudhary BL, Shukla S. Hematological profile among pulmonary tuberculosis patients in tertiary care hospital. Int J Bioassays 2015;4:3900 2.
Shah AR, Desai KN, Maru AM. Evaluation of hematological parameters in pulmonary tuberculosis patients. Journal of Family Medicine and Primary Care. 2022;11.8: 4424-4428.
Campbell EA, Korzheva N, Mustaev A, et al. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell. 2001;104:901–912.
Swain SS, Sharma D, Hussain T, Pati S. Molecular mechanisms of underlying genetic factors and associated mutations for drug resistance in Mycobacterium tuberculosis. Emerg Microbes Infect. 2020;9:1651–1663. doi:10.1080/22221751.2020.1785334
Jamieson FB, Guthrie JL, Neemuchwala A, Lastovetska O, Melano RG, Mehaffy C. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J Clin Microbiol. 2014;52:2157–2162. doi:10.1128/JCM.00691-14
Campbell PJ, Morlock GP, Sikes RD, et al. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2011;55:2032–2041. doi:10.1128/AAC.01550-10
Zaw MT, Emran NA, Lin Z. Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosis. J Infect Public Health. 2018;11:605–610. doi:10.1016/j.jiph.2018.04.005
Williams DL, Spring L, Collins L, et al. Contribution of rpoB mutations to development of rifamycin cross-resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998; 42:1853–1857. doi:10.1128/AAC.42.7.1853
Ali A, Hasan Z, McNerney R, Mallard K, Hill-Cawthorne G, Coll F, et al. Whole-genome sequencing based characterization of extensively drug-resistant Mycobacterium tuberculosis isolates from Pakistan. PLoS One. 2015;10(2):e0117771. doi: 10.1371/journal.pone.0117771.
Sinha P, Srivastava GN, Tripathi R et al. Detection of mutations in the rpoB gene of rifampicin-resistant Mycobacterium tuberculosis strains inhibiting wild type probe hybridization in the MTBDR plus assay by DNA sequencing directly from clinical specimens. BMC Microbiol 20, 284 (2020). https://doi.org/10.1186/s12866-020-01967-5
Sandler T. "The analytical study of terrorism: Taking stock." Journal of Peace Research 51.2 (2014): 257-271.
Saitou N, Nei M, The neighbor-joining method: a new method for reconstructing phylogenetic trees., Molecular Biology and Evolution, Volume 4, Issue 4, Jul 1987, Pages 406–425, https://doi.org/10.1093/oxfordjournals.molbev.a040454
Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016 Jul;33(7):1870-4. doi: 10.1093/molbev/msw054. Epub 2016 Mar 22. PMID: 27004904; PMCID: PMC8210823.
Rohini K, Surekha M, Srikumar PS, Kumar AM. Assessment of hematological parameters in pulmonary tuberculosis patients. Indian J Clin Biochem2016;31:332 5.
Jagielski T, Bakula Z, Brzostek A, et al. Characterization of mutations conferring resistance to rifampin in Mycobacterium tuberculosis clinical strains. Antimicrob Agents Chemother. 2018;62:e01093–18. doi:10.1128/AAC.01093-18
Rajesh HS, Sangeetha B, Indhu S, Manimaran, Nishanth M. Evaluation of hematological profile in pulmonary tuberculosis. Indian J Pathol Oncol 2020;7:39 42
Ciglenecki I, Glynn JR, Mwinga A, Ngwira B, Zumla A, Fine PE, et al. Population differences in death rates in HIV positive patients with tuberculosis. Int J Tuberc Lung Dis 2007;11:1121 8.
Bashir AB, Ageep Ali K, Abufatima AS, Mohamedani AA. Reactive thrombocytosis and ESR in patients with pulmonary tuberculosis. J Clin Pathol2014;5:29 34
Thatoi PK. Pulmonary tuberculosis and its haematological correlates. Trans World Med J 2013;1:11 3. 13. Banerjee M, Chaudhary BL, Shukla S. Hematological profile among pulmonary tuberculosis patients in tertiary care hospital. Int J Bioassays 2015;4:3900 2.
Cavusoglu C, Hilmioglu S, Guneri S, Bilgic A. Characterization of rpoB mutations in rifampin-resistant clinical isolates of Mycobacterium tuberculosis from Turkey by DNA sequencing and line probe assay. J Clin Microbiol. 2002 Dec;40(12):4435-8. doi: 10.1128/JCM.40.12.4435-4438.2002. PMID: 12454132; PMCID: PMC154651.
Billington OJ, McHugh TD, Gillespie SH. Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1999; 41:1866–1869.
Mani C, Selvakumar N, Narayanan S, Narayanan PR. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis clinical isolates from India. J. Clin. Microbiol. 2001; 39:2987–2990.
Williams DL, Wanguespack C, Eisanach K, Crawford J T, Porteals F, Salfinger M, Nolan C M, Abe C, Sticht-Groh V, Gillis TP. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob. Agents Chemother. 1994; 38:2380–2386.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Abdulrazzaq Neamah Zghair

This work is licensed under a Creative Commons Attribution 4.0 International License.
















